[fusion_builder_container admin_label=”Anchor – Our Sceince” hundred_percent=”yes” equal_height_columns=”no” menu_anchor=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”rgba(139,195,74,0)” background_image=”” background_position=”center center” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”” video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”” video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_menu_anchor name=”science” class=”” /][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Container – Our Science” hundred_percent=”yes” equal_height_columns=”yes” menu_anchor=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”0px” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”0″ padding_right=”0″ padding_bottom=”0″ padding_left=”0″ admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”2_3″ layout=”2_3″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
Science
[/fusion_text][fusion_text]
Developing Therapeutics for Cancer and Rare Diseases
[/fusion_text][fusion_text]
LAM Therapeutics develops precision therapeutics for cancer and rare diseases. Our aim is to accelerate drug development by deploying technologies including next-gen sequencing, genome editing, chemical genomics, and combinational drug screening, with deep learning to match clinical drugs to new indications. LAM has advanced three drugs into clinical trials: LAM-001 for lymphangioleiomyomatosis, LAM-002 for B-cell non-Hodgkin lymphoma, and LAM-003 for acute myeloid leukemia.
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”1_3″ layout=”1_3″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#415b86″ background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/aritificial-intelligence-web-02-1024×695.jpg” background_position=”center top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”right” animation_speed=”0.7″ animation_offset=”” last=”no” element_content=””][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container hundred_percent=”yes” overflow=”visible” admin_toggled=”yes” admin_label=”Anchor – Approach”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_menu_anchor name=”approach” class=”” /][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Container – Our Approach” hundred_percent=”no” equal_height_columns=”no” menu_anchor=”” hide_on_mobile=”no” class=”” id=”” background_color=”#f4f6f8″ background_image=”https://lamtherapeutics.com/wp-content/uploads/2015/10/light_gradient_bg.jpg” background_position=”center center” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”0px” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”1.5%” padding_right=”” padding_bottom=”2.5%” padding_left=”” admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”1_6″ layout=”1_5″ last=”no” spacing=”yes” center_content=”no” hide_on_mobile=”yes” background_color=”” background_image=”” background_repeat=”no-repeat” background_position=”left top” hover_type=”none” link=”” border_position=”all” border_size=”0px” border_color=”” border_style=”solid” padding=”” margin_top=”” margin_bottom=”” animation_type=”0″ animation_direction=”down” animation_speed=”0.1″ animation_offset=”” class=”” id=”” element_content=”” min_height=””][/fusion_builder_column][fusion_builder_column type=”2_3″ layout=”3_5″ last=”no” spacing=”yes” center_content=”no” hide_on_mobile=”no” background_color=”” background_image=”” background_repeat=”no-repeat” background_position=”left top” hover_type=”none” link=”” border_position=”all” border_size=”0px” border_color=”” border_style=”solid” padding=”” margin_top=”65px” margin_bottom=”” animation_type=”0″ animation_direction=”down” animation_speed=”0.1″ animation_offset=”” class=”” id=”” min_height=””][fusion_text]
Approach
[/fusion_text][fusion_text]
LAM’s virtuous circle strategy integrates patient data, AI, and next-generation sequencing. We test LAM drugs in clinical trials, and then analyze genomics and proteomics data to match drugs to patients. Our deep learning AI technology gets smarter with each patient treated and more data analyzed, which helps us select the most appropriate patients to treat.
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”1_6″ layout=”1_5″ last=”yes” spacing=”yes” center_content=”no” hide_on_mobile=”yes” background_color=”” background_image=”” background_repeat=”no-repeat” background_position=”left top” hover_type=”none” link=”” border_position=”all” border_size=”0px” border_color=”” border_style=”solid” padding=”” margin_top=”” margin_bottom=”” animation_type=”0″ animation_direction=”down” animation_speed=”0.1″ animation_offset=”” class=”” id=”” element_content=”” min_height=””][/fusion_builder_column][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_content_boxes layout=”clean-vertical” columns=”3″ title_size=”32px” title_color=”” body_color=”” backgroundcolor=”” iconcolor=”#31b6e3″ icon_circle=”” icon_circle_radius=”round” circlecolor=”” circlebordersize=”0px” circlebordercolor=”” outercirclebordersize=”0″ outercirclebordercolor=”transparent” icon_size=”” icon_hover_type=”none” hover_accent_color=”#515b69″ link_type=”” link_area=”link-icon” link_target=”” icon_align=”left” animation_type=”fade” animation_delay=”300″ animation_offset=”” animation_direction=”down” animation_speed=”0.7″ margin_top=”0px” margin_bottom=”0px” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=””][fusion_content_box title=”Patient Data” backgroundcolor=”” icon=”fa-phone” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”” circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/patient-data.png” image_width=”150″ image_height=”150″ link=”” linktext=”” link_target=”” animation_type=”none” animation_direction=”down” animation_speed=”0.7″ animation_offset=”” /][fusion_content_box title=”Artificial Intelligence” backgroundcolor=”” icon=”fa-comment” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”#31b6e3″ circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/AI-icon-blue-02-01.png” image_width=”150″ image_height=”150″ link=”” linktext=”” link_target=”” animation_type=”none” animation_direction=”down” animation_speed=”0.7″ animation_offset=”” /][fusion_content_box title=”Next-gen Sequencing” backgroundcolor=”” icon=”fa-comment” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”#31b6e3″ circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/DNA-icon-03.png” image_width=”150″ image_height=”150″ link=”” linktext=”” link_target=”” animation_type=”none” animation_direction=”down” animation_speed=”0.7″ animation_offset=”” /][/fusion_content_boxes][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container hundred_percent=”yes” overflow=”visible” admin_toggled=”yes” admin_label=”Anchor – Publications”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_menu_anchor name=”publications” class=”” /][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Container – Publications” hundred_percent=”no” equal_height_columns=”no” menu_anchor=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”center center” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”0px” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”1.5%” padding_right=”” padding_bottom=”2.5%” padding_left=”” admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”3_5″ layout=”3_5″ spacing=”yes” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”65px” margin_bottom=”” animation_type=”” animation_direction=”down” animation_speed=”0.1″ animation_offset=”” last=”no”][fusion_text]
Publications
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”1_5″ layout=”1_5″ last=”no” spacing=”yes” center_content=”no” hide_on_mobile=”yes” background_color=”” background_image=”” background_repeat=”no-repeat” background_position=”left top” hover_type=”none” link=”” border_position=”all” border_size=”0px” border_color=”” border_style=”solid” padding=”” margin_top=”” margin_bottom=”” animation_type=”0″ animation_direction=”down” animation_speed=”0.1″ animation_offset=”” class=”” id=”” element_content=”” min_height=””][/fusion_builder_column][fusion_builder_column type=”1_5″ layout=”1_5″ last=”yes” spacing=”yes” center_content=”no” hide_on_mobile=”yes” background_color=”” background_image=”” background_repeat=”no-repeat” background_position=”left top” hover_type=”none” link=”” border_position=”all” border_size=”0px” border_color=”” border_style=”solid” padding=”” margin_top=”” margin_bottom=”” animation_type=”0″ animation_direction=”down” animation_speed=”0.1″ animation_offset=”” class=”” id=”” element_content=”” min_height=””][/fusion_builder_column][fusion_builder_column type=”1_4″ layout=”1_4″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”3_4″ layout=”3_4″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
Phase 1 Clinical Safety, Pharmacokinetics (PK), and Activity of Apilimod Dimesylate (LAM-002A), a First-in-Class Inhibitor of Phosphatidylinositol-3-Phosphate 5-Kinase (PIKfyve), in Patients with Relapsed or Refractory B-Cell Malignancies
Wael A Harb, Catherine S Diefenbach, Nehal Lakhani, Sarah C Rutherford, Marshall T Schreeder, Stephen M. Ansell, Taimur Sher, David M Aboulafia, Jonathon B Cohen, Darrell Nix, Sean Landrette, Kate Flanders, Langdon L Miller, Henri Lichenstein and Jeremy S. Abramson
59th ASH Annual Meeting & Exposition
[/fusion_text][fusion_separator style_type=”single solid” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” sep_color=”” top_margin=”” bottom_margin=”” border_size=”” icon=”” icon_circle=”” icon_circle_color=”” width=”” alignment=”center” /][/fusion_builder_column][fusion_builder_column type=”1_4″ layout=”1_4″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”3_4″ layout=”3_4″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
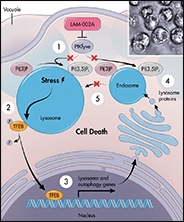
Identification of apilimod as a first-in-class PIKfyve kinase inhibitor for treatment of B-cell non-Hodgkin lymphoma
Sophia Gayle, Sean Landrette, Neil Beeharry, Chris Conrad, Marylens Hernandez, Paul Beckett, Shawn M. Ferguson, Talya Mandelkern, Meiling Zheng, Tian Xu, Jonathan Rothberg and Henri Lichenstein
View publication
View supplemental methods, figures, and data
[/fusion_text][fusion_separator style_type=”single solid” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” sep_color=”” top_margin=”” bottom_margin=”” border_size=”” icon=”” icon_circle=”” icon_circle_color=”” width=”” alignment=”center” /][/fusion_builder_column][fusion_builder_column type=”1_4″ layout=”1_4″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text] [/fusion_text][/fusion_builder_column][fusion_builder_column type=”3_4″ layout=”3_4″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”3_4″ layout=”3_4″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
B-cell non-Hodgkin lymphoma: Selective vulnerability to PIKFYVE inhibition
Sophia Gayle, Sean Landrette, Neil Beeharry, Chris Conrad, Marylens Hernandez, Paul Beckett, Shawn M. Ferguson, Talya Mandelkern, Meiling Zheng, Tian Xu, Jonathan Rothberg and Henri Lichenstein
AUTOPHAGY 2017, VOL. 13, NO. 6, 1–2
[/fusion_text][fusion_separator style_type=”single solid” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” sep_color=”” top_margin=”” bottom_margin=”” border_size=”” icon=”” icon_circle=”” icon_circle_color=”” width=”” alignment=”center” /][/fusion_builder_column][fusion_builder_column type=”1_4″ layout=”1_4″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”3_4″ layout=”3_4″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#ffffff” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
Toward autophagy-targeted therapy in lymphoma
Lapo Alinari
[/fusion_text][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Parallax 02″ hundred_percent=”no” equal_height_columns=”no” menu_anchor=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Digital-DNA-background-web.jpg” background_position=”left center” background_repeat=”no-repeat” fade=”no” background_parallax=”up” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”150px” padding_right=”” padding_bottom=”150px” padding_left=”” admin_toggled=”yes”][fusion_builder_row][/fusion_builder_row][/fusion_builder_container][fusion_builder_container hundred_percent=”yes” overflow=”visible” admin_toggled=”yes” admin_label=”Anchor – Programs”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_menu_anchor name=”programs” class=”” /][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Container – Our Programs” hundred_percent=”no” equal_height_columns=”no” menu_anchor=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”0px” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”5%” padding_right=”” padding_bottom=”6%” padding_left=”” admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”1_2″ layout=”1_2″ spacing=”yes” center_content=”no” link=”” target=”_self” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” hover_type=”none” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”35px” margin_bottom=”0px” animation_type=”” animation_direction=”down” animation_speed=”0.1″ animation_offset=”” last=”no”][fusion_imageframe image_id=”733″ style_type=”none” stylecolor=”” hover_type=”none” bordersize=”0px” bordercolor=”” borderradius=”0″ align=”center” lightbox=”no” gallery_id=”” lightbox_image=”” alt=”” link=”” linktarget=”_self” hide_on_mobile=”no” class=”” id=”” animation_type=”” animation_direction=”up” animation_speed=”0.7″ animation_offset=””]https://lamtherapeutics.com/wp-content/uploads/2017/06/LAM-Logo-Circle-02.png[/fusion_imageframe][/fusion_builder_column][fusion_builder_column type=”1_2″ layout=”1_2″ spacing=”yes” center_content=”no” link=”” target=”_self” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” hover_type=”none” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”20px” margin_bottom=”” animation_type=”” animation_direction=”up” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
Precision Medicine Programs
[/fusion_text][fusion_text]
Accelerating discovery of medicines to change the lives of people we love
[/fusion_text][fusion_text]
Our programs target lymphangioleimyomatosis, B-cell non-Hodgkin lymphoma and acute myeloid leukemia.
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_separator style_type=”none” top_margin=”30″ alignment=”center” /][/fusion_builder_column][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_menu_anchor name=”programs1-3″ class=”” /][fusion_content_boxes layout=”icon-on-side” columns=”3″ title_size=”28px” title_color=”#050505″ body_color=”#0f0f0f” backgroundcolor=”” icon=”” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”” icon_circle=”” icon_circle_radius=”” circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” icon_size=”” icon_hover_type=”none” hover_accent_color=”#7f8795″ image=”” image_width=”” image_height=”” link_type=”” link_area=”link-icon” link_target=”” icon_align=”left” animation_type=”fade” animation_delay=”350″ animation_offset=”” animation_direction=”left” animation_speed=”0.7″ margin_top=”” margin_bottom=”2%” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=””][fusion_content_box title=”LAM-001, Inhaled mTOR Kinase Inhibitor” backgroundcolor=”” icon=”fa-power-off” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”” circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Lung-icon.png” image_width=”75″ image_height=”75″ link=”” linktext=”” link_target=”” animation_type=”fade” animation_direction=”left” animation_speed=”0.7″ animation_offset=””]
Hyperactivation of mTOR signaling is a central driver in the genetic-based disease lymphangioleiomyomatosis that is found primarily in women and often affects other organs including lymph nodes and kidneys. The Phase 1 trial of LAM-001, inhaled mTOR inhibitor developed to treat lymphangioleimyomatosis has been completed.
[/fusion_content_box][fusion_content_box title=”LAM-002, PIKfyve Kinase Inhibitor” backgroundcolor=”” icon=”fa-wrench” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”” circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/LAM-capsule.png” image_width=”75″ image_height=”75″ link=”” linktext=”” link_target=”” animation_type=”fade” animation_direction=”left” animation_speed=”0.7″ animation_offset=””]
LAM-002 is an exquisitely selective first-in-class PIKfyve kinase inhibitor that kills specific cancers and has little to no effect on normal cells. LAM-002 has completed the dose escalation portion of a Phase 1 trial in relapsed or refractory B-cell non-Hodgkin lymphoma, and a recommended Phase 2 dose has been selected. LAM-002 was found to be safe and well-tolerated, and anti-tumor activity was observed in patients who had failed multiple prior lines of therapy. LAM-002 is now being evaluated as single agent and in combination with either atezolizumab or rituximab in specific lymphoma subtypes.
[/fusion_content_box][fusion_content_box title=”LAM-003, Immune-modulating, Anti-cancer Drug” backgroundcolor=”” icon=”fa-bell” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”” circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/LAM-capsule.png” image_width=”75″ image_height=”75″ link=”” linktext=”” link_target=”” animation_type=”fade” animation_direction=”left” animation_speed=”0.7″ animation_offset=””]
LAM-003 is an orally active first-in-class small molecule immuno-modulatory drug for a genomically-defined population of acute myeloid leukemia patients. LAM-003 has potent anti-leukemic activity in preclinical models and a Phase 1 trial of LAM-003 has been initiated.
[/fusion_content_box][/fusion_content_boxes][/fusion_builder_column][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_content_boxes layout=”icon-on-side” columns=”3″ title_size=”28px” title_color=”#050505″ body_color=”#0f0f0f” backgroundcolor=”” icon=”” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”” icon_circle=”” icon_circle_radius=”” circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” icon_size=”” icon_hover_type=”none” hover_accent_color=”#7f8795″ image=”” image_width=”” image_height=”” link_type=”” link_area=”link-icon” link_target=”” icon_align=”left” animation_type=”fade” animation_delay=”350″ animation_offset=”” animation_direction=”left” animation_speed=”0.7″ margin_top=”15px” margin_bottom=”15px” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=””][fusion_content_box title=”Other Programs” backgroundcolor=”” icon=”fa-toggle-on” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”” circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/LAM-capsule.png” image_width=”75″ image_height=”75″ link=”” linktext=”” link_target=”” animation_type=”fade” animation_direction=”left” animation_speed=”0.7″ animation_offset=””]
LAM Therapeutics is actively applying its technologies to advance discovery of clinical stage drugs for immuno-oncology and rare disease indications.
[/fusion_content_box][fusion_content_box title=”Clinical Trials” backgroundcolor=”” icon=”fa-refresh” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”” circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/LAM-data.png” image_width=”75″ image_height=”75″ link=”#trials” linktext=”” link_target=”” animation_type=”fade” animation_direction=”left” animation_speed=”0.7″ animation_offset=””]
Learn more about LAM Therapeutics clinical trials >
[/fusion_content_box][fusion_content_box title=”Publications” backgroundcolor=”” icon=”fa-inbox” iconflip=”” iconrotate=”” iconspin=”no” iconcolor=”” circlecolor=”” circlebordersize=”” circlebordercolor=”” outercirclebordersize=”” outercirclebordercolor=”” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/LAM-publications.png” image_width=”75″ image_height=”75″ link=”#publications” linktext=”” link_target=”” animation_type=”fade” animation_direction=”left” animation_speed=”0.7″ animation_offset=””]
View LAM Therapeutics publications >
[/fusion_content_box][/fusion_content_boxes][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container hundred_percent=”yes” overflow=”visible” admin_toggled=”yes” admin_label=”Anchor – Clinical Trials”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_menu_anchor name=”trials” class=”” /][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Container – Clinical Trials” hundred_percent=”no” equal_height_columns=”no” menu_anchor=”” hide_on_mobile=”no” class=”” id=”” background_color=”#f4f6f8″ background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/LAM-clinical-trials.jpg” background_position=”center bottom” background_repeat=”no-repeat” fade=”no” background_parallax=”up” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”0px” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”2%” padding_right=”” padding_bottom=”0px” padding_left=”” admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”2_3″ layout=”2_3″ spacing=”yes” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 0px 11%” margin_top=”65px” margin_bottom=”” animation_type=”” animation_direction=”down” animation_speed=”0.1″ animation_offset=”” last=”no”][fusion_text]
Clinical Trials
[/fusion_text][fusion_text]A clinical trial is a carefully supervised research study performed in humans before an investigational drug is available to the general public. Clinical trials play an important role in the development process of new drugs by providing data to evaluate a drug’s safety and efficacy. Anyone interested in participating in a clinical study should know as much as possible about the study and discuss any questions or concerns with their physician and healthcare providers in order to make an informed decision prior to enrolling in a clinical trial. Learn more about clinical trials.[/fusion_text][/fusion_builder_column][fusion_builder_column type=”1_3″ layout=”1_6″ last=”yes” spacing=”yes” center_content=”no” hide_on_mobile=”yes” background_color=”” background_image=”” background_repeat=”no-repeat” background_position=”left top” hover_type=”none” link=”” border_position=”all” border_size=”0px” border_color=”” border_style=”solid” padding=”” margin_top=”” margin_bottom=”” animation_type=”0″ animation_direction=”down” animation_speed=”0.1″ animation_offset=”” class=”” id=”” element_content=”” min_height=””][/fusion_builder_column][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_separator style_type=”none” top_margin=”50″ alignment=”center” /][/fusion_builder_column][fusion_builder_column type=”1_1″ layout=”1_1″ spacing=”yes” center_content=”no” hover_type=”none” link=”” min_height=”none” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”” border_color=”” border_style=”solid” border_position=”all” padding=”0px 11% 11% 11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no”][fusion_text]
To learn more about LAM Therapeutics clinical trials, please select a specific trial.
[/fusion_text][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container hundred_percent=”yes” overflow=”visible” admin_toggled=”yes” admin_label=”Anchor – Careers”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_menu_anchor name=”careers” class=”” /][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Container – Careers” hundred_percent=”yes” equal_height_columns=”yes” menu_anchor=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”0px” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”0″ padding_right=”0″ padding_bottom=”0″ padding_left=”0″ admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”2_3″ layout=”2_3″ spacing=”no” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#37507a” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”11%” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.7″ animation_offset=”” last=”no”][fusion_text]
Careers
[/fusion_text][fusion_text]
LAM Therapeutics is a fully-funded startup dedicated to developing breakthrough therapeutics for the treatment of rare diseases and cancer.
Working with proprietary technologies, LAM Therapeutics is making a difference. The company is privately funded, allowing you to focus 100% on doing the best science of your life.
Join a world-class team in developing the next-generation of breakthrough drugs. LAM Therapeutics is seeking extraordinary gifted and driven scientists, with a track record of success developing, implementing and scaling up new technologies.
[/fusion_text][fusion_code]Jmx0O3NjcmlwdCB0eXBlPSYjeDI3O3RleHQvamF2YXNjcmlwdCYjeDI3OyBzcmM9JiN4Mjc7aHR0cHM6Ly9zdGF0aWMuc21hcnRyZWNydWl0ZXJzLmNvbS9qb2Itd2lkZ2V0LzEuMy4zL3NjcmlwdC9zbWFydF93aWRnZXQuanMmI3gyNzsmZ3Q7Jmx0Oy9zY3JpcHQmZ3Q7CiZsdDtzY3JpcHQgdHlwZT0mI3gyNzt0ZXh0L2phdmFzY3JpcHQmI3gyNzsgY2xhc3M9JiN4Mjc7am9iX3dpZGdldCYjeDI3OyZndDsKd2lkZ2V0KHsKICAgICZxdW90O2NvbXBhbnlfY29kZSZxdW90OzogJnF1b3Q7NENhdGFseXplciZxdW90OywKICAgICZxdW90O2JnX2NvbG9yX3dpZGdldCZxdW90OzogJnF1b3Q7I2ZmZmZmZiZxdW90OywKICAgICZxdW90O2JnX2NvbG9yX2hlYWRlcnMmcXVvdDs6ICZxdW90OyM5Njk2OTYmcXVvdDssCiAgICAmcXVvdDtiZ19jb2xvcl9saW5rcyZxdW90OzogJnF1b3Q7I2ZmNjY5OSZxdW90OywKICAgICZxdW90O3R4dF9jb2xvcl9oZWFkZXJzJnF1b3Q7OiAmcXVvdDsjMjkyOTI5JnF1b3Q7LAogICAgJnF1b3Q7dHh0X2NvbG9yX2pvYiZxdW90OzogJnF1b3Q7IzNkM2QzZCZxdW90OywKICAgICZxdW90O2JnX2NvbG9yX2V2ZW5fcm93JnF1b3Q7OiAmcXVvdDsjZTBlMGUwJnF1b3Q7LAogICAgJnF1b3Q7YmdfY29sb3Jfb2RkX3JvdyZxdW90OzogJnF1b3Q7I2Y3ZjdmNyZxdW90OywKICAgICZxdW90O2F1dG9fd2lkdGgmcXVvdDs6ICZxdW90O2F1dG8mcXVvdDssCiAgICAmcXVvdDthdXRvX2hlaWdodCZxdW90OzogJnF1b3Q7YXV0byZxdW90OywKICAgICZxdW90O251bWJlciZxdW90OzogJnF1b3Q7b24mcXVvdDssCiAgICAmcXVvdDtqb2JfdGl0bGUmcXVvdDs6ICZxdW90O3RydWUmcXVvdDssCiAgICAmcXVvdDtsb2NhdGlvbiZxdW90OzogJnF1b3Q7dHJ1ZSZxdW90OywKICAgICZxdW90O2ZpbHRlcl9kZXBhcnRtZW50cyZxdW90OzogJnF1b3Q7TEFNJnF1b3Q7LAogICAgJnF1b3Q7am9iQWRUeXBlJnF1b3Q7OiAmcXVvdDtQVUJMSUMmcXVvdDssCiAgICAmcXVvdDt0cmlkJnF1b3Q7OiAmcXVvdDsmcXVvdDssCiAgICAmcXVvdDthcGlfdXJsJnF1b3Q7OiAmcXVvdDtodHRwczovL3d3dy5zbWFydHJlY3J1aXRlcnMuY29tJnF1b3Q7LAogICAgJnF1b3Q7Y3VzdG9tX2Nzc191cmwmcXVvdDs6ICZxdW90O2h0dHBzOi8vc3RhdGljLnNtYXJ0cmVjcnVpdGVycy5jb20vam9iLXdpZGdldC8xLjMuMy9jc3Mvc21hcnRfd2lkZ2V0LmNzcyZxdW90Owp9KTsKJmx0Oy9zY3JpcHQmZ3Q7[/fusion_code][fusion_separator style_type=”none” top_margin=”50″ alignment=”center” /][fusion_text]
NOTE TO RECRUITERS
The Company does not accept resumes submitted by, or candidates referred from, external agencies by any means (including but not limited to via the Internet, email, fax, U.S. mail and/or verbal communications). Any third-party resume forwarded by such recruiters will be treated as a direct application and shall be deemed to be the property of the Company, and the Company reserves the right to contact the candidate directly and the recruiter will not receive any compensation from the Company.
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”1_3″ layout=”1_3″ spacing=”no” center_content=”no” link=”” target=”_self” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”#415b86″ background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/DSC_0954-copy-copy.jpg” background_position=”center top” undefined=”” background_repeat=”no-repeat” hover_type=”none” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”right” animation_speed=”0.7″ animation_offset=”” last=”no”][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Team Photos” hundred_percent=”yes” equal_height_columns=”yes” menu_anchor=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”center center” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”” border_color=”” border_style=”solid” margin_top=”0″ margin_bottom=”0″ padding_top=”0px” padding_right=”0px” padding_bottom=”0px” padding_left=”0px” admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”1_3″ layout=”1_3″ spacing=”0%” center_content=”no” hover_type=”zoomin” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/DSC_1158-copy-copy-1024×684.jpg” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 0px 0px 0px” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” element_content=””][/fusion_builder_column][fusion_builder_column type=”1_3″ layout=”1_3″ spacing=”0%” center_content=”no” hover_type=”zoomin” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/DSC_1067-copy-copy-1024×684.jpg” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 0px 0px 0px” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” element_content=””][/fusion_builder_column][fusion_builder_column type=”1_3″ layout=”1_3″ spacing=”0%” center_content=”no” hover_type=”zoomin” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/DSC_1108-copy-copy-1024×684.jpg” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 0px 0px 0px” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” element_content=””][/fusion_builder_column][fusion_builder_column type=”1_3″ layout=”1_3″ spacing=”0%” center_content=”no” hover_type=”zoomin” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/DSC_1148-copy-copy-02-1024×684.jpg” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 0px 0px 0px” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” element_content=””][/fusion_builder_column][fusion_builder_column type=”1_3″ layout=”1_3″ spacing=”0%” center_content=”no” link=”” target=”_self” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/11/DSC_0985_1920x1080-landscape02.jpg” background_position=”left top” undefined=”” background_repeat=”no-repeat” hover_type=”zoomin” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding_top=”0px” padding_right=”0px” padding_bottom=”0px” padding_left=”0px” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no”][/fusion_builder_column][fusion_builder_column type=”1_3″ layout=”1_3″ spacing=”0%” center_content=”no” hover_type=”zoomin” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/DSC_0945-copy-copy-1024×684.jpg” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”0px 0px 0px 0px” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” element_content=””][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container hundred_percent=”yes” overflow=”visible” admin_toggled=”yes” admin_label=”Anchor – About”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_menu_anchor name=”about” class=”” /][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Container – About LAM” hundred_percent=”no” equal_height_columns=”no” menu_anchor=”” hide_on_mobile=”no” class=”” id=”” background_color=”#f4f6f8″ background_image=”https://lamtherapeutics.com/wp-content/uploads/2015/10/light_gradient_violet_bg.jpg” background_position=”center bottom” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”0px” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”0px” padding_right=”” padding_bottom=”0px” padding_left=”” admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ spacing=”yes” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”11% 0px 0px 0px” margin_top=”” margin_bottom=”” animation_type=”” animation_direction=”down” animation_speed=”0.1″ animation_offset=”” last=”no”][fusion_text]
About LAM Therapeutics
[/fusion_text][fusion_separator style_type=”none” top_margin=”50″ alignment=”center” /][fusion_testimonials design=”classic” backgroundcolor=”” textcolor=”#0a0a0a” random=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=””][fusion_testimonial name=”Dr. Tian Xu” avatar=”male” image=”” image_border_radius=”” company=”LAM co-founder” link=”” target=”_self”]
LAM is at the forefront of transforming how we match drugs to patients for best response. We call our strategy the ‘virtuous circle.’ We test LAM drugs in patients, and then analyze the data with genomics and proteomics. Our deep learning AI technology gets smarter with each patient treated and more data analyzed, which helps us select the most appropriate patients to treat.
[/fusion_testimonial][/fusion_testimonials][/fusion_builder_column][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_separator style_type=”none” top_margin=”50″ alignment=”center” /][/fusion_builder_column][fusion_builder_column type=”1_1″ layout=”1_1″ spacing=”yes” center_content=”no” hover_type=”none” link=”” min_height=”none” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”” border_color=”” border_style=”solid” border_position=”all” padding=”0px 0px 11% 0px” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no”][fusion_menu_anchor name=”people” class=”” /][fusion_text]
Our People
[/fusion_text][fusion_flip_boxes columns=”4″ icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”” image_width=”” image_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=””][fusion_flip_box title_front=”Henri Lichenstein, Ph.D.” title_back=”Henri Lichenstein, Ph.D.” text_front=”President & CEO” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”yes” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Henri-Lichenstein-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Henri Lichenstein is the President and CEO of LAM Therapeutics. Dr. Lichenstein has over 30 years of drug development experience at Amgen, CuraGen Corporation, Topotarget, and BioPontis Alliance, and has led numerous successful drug development efforts resulting in the advancement of therapeutics from discovery into clinical development. At Topotarget and CuraGen Corporation, Dr. Lichenstein led the global pre- and clinical development of the histone deacetylase inhibitor, belinostat, in…
[fusion_modal_text_link name=”Henri” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”Tian Xu, Ph. D.” title_back=”Tian Xu, Ph. D.” text_front=”Scientific Advisory Board” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Tian-Xu-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Tian Xu serves on LAM Therapeutics Scientific Advisory Board. Dr. Xu is Professor and Vice Chair of Genetics at Yale University School of Medicine and a Howard Hughes Medical Institute Investigator. He also serves as Director of the Institute of Developmental Biology and Molecular Medicine at Fudan University in Shanghai. He was President of the Chinese Biological Investigators Society and Chair of the Scientific Advisory Board for the Rothberg Institute for Childhood Diseases. The major thrust of…
[fusion_modal_text_link name=”Tian” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”Peter Young, Ph.D.” title_back=”Peter Young, Ph.D.” text_front=”Head of Research and Translational Sciences” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/PeterYoung-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Peter Young is the Head of Research and Translational Sciences at LAM Therapeutics. Dr. Young has over 25 years of experience in target identification, validation, drug discovery and development while holding various executive and management positions at SmithKline Beecham, DuPont Pharmaceuticals, SUGEN, Celera, Genelabs, Affymax and Resverlogix. He has worked in multiple therapeutic areas such as oncology, autoimmune/inflammation, hematology, metabolic and anti-viral disease…
[fusion_modal_text_link name=”Peter” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”Robert Spiegel, M.D. FACP” title_back=”Robert Spiegel, M.D. FACP” text_front=”Scientific Advisory Board” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Robert-Spiegel-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Robert Spiegel joined the LAM Therapeutics Scientific Advisory Board in April, 2013. Dr. Spiegel is the founder of Spiegel Consulting LLC, an independent advisory firm to pharmaceutical and biotechnology companies, and serves as a Senior Advisor to Warburg Pincus and BioPontis Alliance. He retired in 2009 as Chief Medical Officer at Schering-Plough. With 25+ years experience in the pharmaceutical industry, he played…
[fusion_modal_text_link name=”Robert” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”Brendan Manning, Ph.D.” title_back=”Brendan Manning, Ph.D.” text_front=”Scientific Advisory Board” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Brendan-Manning-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Brendan Manning joined the LAM Therapeutics Scientific Advisory Board in May, 2013. Dr. Manning is a Professor in the Department of Genetics and Complex Diseases at the Harvard School of Public Health (HSPH). His doctoral training was in the laboratory of Michael Snyder at Yale University, where he worked basic mechanisms of cell division. Following receipt of his Ph.D. in 2000, Dr. Manning joined the laboratory of Lewis Cantley at Harvard Medical School for his postdoctoral research. During this…
[fusion_modal_text_link name=”Brendan” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”Lawrence Melvin, Ph.D.” title_back=”Lawrence Melvin, Ph.D.” text_front=”Scientific Advisory Board” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Lawrence-Melvin-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Larry Melvin joined the LAM Therapeutics Scientific Advisory Board in November, 2013. Dr. Melvin has over 30 years of experience in drug discovery and development with Pfizer, Amgen, Myogen and Gilead. Dr. Melvin has a broad background in medicinal chemistry including the areas of inflammation, respiratory, cardiovascular, central nervous system and antibacterial. He has initiated and led successful drug discovery programs yielding compounds for clinical study and participated in…
[fusion_modal_text_link name=”Lawrence” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”Joel S. Bader, Ph.D.” title_back=”Joel S. Bader, Ph.D.” text_front=”Scientific Advisory Board ” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Joel-Bader-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Joel Bader joined the LAM Therapeutics Scientific Advisory Board in December, 2014. Dr. Bader is an Associate Professor in the Department of Biomedical Engineering, Johns Hopkins University, and is Interim Director of the High-Throughput Biology Center at the Johns Hopkins School of Medicine. He is a member of the Institute of Computational Medicine and the Institute of Genetic Medicine, and he holds a secondary appointment in the Department of Computer Science at Johns Hopkins…
[fusion_modal_text_link name=”Joel” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”Joel Dudley, Ph.D.” title_back=”Joel Dudley, Ph.D.” text_front=”Scientific Advisory Board” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Joel-Dudley-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Joel Dudley joined the LAM Therapeutics Scientific Advisory Board in December, 2014. Dr. Dudley is Assistant Professor of Genetics and Genomic Sciences and Director of Biomedical Informatics at the Icahn School of Medicine at Mount Sinai in New York City. Dr. Dudley obtained his PhD in Biomedical Informatics from Stanford University School of Medicine, where he developed research in the areas of genomic medicine and translational bioinformatics. Prior to Mount Sinai, he held positions as Co-founder…
[fusion_modal_text_link name=”JoelDudley” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”Paul Beckett, Ph.D.” title_back=”Paul Beckett, Ph.D.” text_front=”Senior Director, Drug Discovery” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Paul-Beckett-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Paul Beckett joined LAM Therapeutics as Senior Director, Drug Discovery in August, 2013. He is an internationally experienced medicinal chemist and drug discovery leader with a background in emerging biotech/pharmaceutical companies. He played a prominent role in the establishment of British Biotech Pharmaceuticals, the UK’s first publicly traded biotech company, where he contributed extensively to the discovery of the matrix metalloproteinase inhibitor BB-2516 (Marimastat, reached…
[fusion_modal_text_link name=”Paul” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”Jonathan Rothberg, Ph.D.” title_back=”Jonathan Rothberg, Ph.D.” text_front=”Board of Directors” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Jonathan-Rothberg-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Inventor of High-Speed DNA Sequencing. Founder Ion Torrent, RainDance, 454 Life Sciences, ClariFi, and CuraGen.
[fusion_modal_text_link name=”Rothberg” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”Gregg Fergus” title_back=”Gregg Fergus” text_front=”Board of Directors” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/Gregg-Fergus-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Gregg Fergus is the Chief Executive Officer and Chief Commercial Officer of 4Catalyzer, a Guilford, Connecticut-based startup accelerator dedicated to democratizing healthcare and maximizing…
[fusion_modal_text_link name=”Fergus” class=”” id=””]View full profile >[/fusion_modal_text_link][/fusion_flip_box][fusion_flip_box title_front=”George Rehm” title_back=”George Rehm” text_front=”Board of Directors” background_color_front=”” title_front_color=”” text_front_color=”” background_color_back=”” title_back_color=”” text_back_color=”” border_size=”” border_color=”” border_radius=”” icon=”” icon_color=”” circle=”” circle_color=”” circle_border_color=”” icon_flip=”” icon_rotate=”” icon_spin=”no” image=”https://lamtherapeutics.com/wp-content/uploads/2017/06/George-Rehm-150-Square.jpg” image_width=”150″ image_height=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=””]
Founding Partner, aeris CAPITAL AG. Advisor to various family offices.
[/fusion_flip_box][/fusion_flip_boxes][fusion_modal name=”Henri” title=”Henri Lichenstein, Ph.D.” size=”large” background=”#efefef” border_color=”” show_footer=”yes” class=”” id=””]
Henri Lichenstein is the President and CEO of LAM Therapeutics. Dr. Lichenstein has over 30 years of drug development experience at Amgen, CuraGen Corporation, Topotarget, and BioPontis Alliance, and has led numerous successful drug development efforts resulting in the advancement of therapeutics from discovery into clinical development. At Topotarget and CuraGen Corporation, Dr. Lichenstein led the global pre- and clinical development of the histone deacetylase inhibitor, belinostat, in over 20 oncology clinical trials, including a registration study in peripheral T-cell lymphoma that led to drug approval by the FDA. Dr. Lichenstein also has significant business development and transaction experience, having closed deals (both in- and out-licensing) and financing worth in excess of $1B. Dr. Lichenstein earned his Ph.D. in Biochemistry and Molecular Biology from the University of California, Santa Barbara and is co-author and inventor on over 50 publications and 60 patents, respectively.
[/fusion_modal][fusion_modal name=”Tian” title=”Tian Xu, Ph.D.” size=”large” background=”” border_color=”” show_footer=”yes” class=”” id=””]Tian Xu serves on LAM Therapeutics Scientific Advisory Board. Dr. Xu is Professor and Vice Chair of Genetics at Yale University School of Medicine and a Howard Hughes Medical Institute Investigator. He also serves as Director of the Institute of Developmental Biology and Molecular Medicine at Fudan University in Shanghai. He was President of the Chinese Biological Investigators Society and Chair of the Scientific Advisory Board for the Rothberg Institute for Childhood Diseases. The major thrust of Xu’s research effort lies in developing and utilizing novel genetic approaches to understand Development and Disease. The systematic functional genetics methods that he developed for Drosophila, mice and human cells have been used by the research community throughout the world. He is also the pioneer and leader for Growth and Size Control and identified the major pathways including TSC/Tor, that regulate growth in development and tumorigenesis. His recent work showing oncogenic cooperation for mutations in different neighboring cells brought new concept and the examination of tumor genetic heterogeneity for cancer biology and therapy.[/fusion_modal][fusion_modal name=”Peter” title=”Peter Young, Ph.D.” size=”large” background=”” border_color=”” show_footer=”yes” class=”” id=””]Peter Young is the Head of Research and Translational Sciences at LAM Therapeutics. Dr. Young has over 25 years of experience in target identification, validation, drug discovery and development while holding various executive and management positions at SmithKline Beecham, DuPont Pharmaceuticals, SUGEN, Celera, Genelabs, Affymax and Resverlogix. He has worked in multiple therapeutic areas such as oncology, autoimmune/inflammation, hematology, metabolic and anti-viral disease and has contributed to the discovery and/or development of Promacta® for Idiopathic Thrombocytopenia Purpura, Imbruvica® for hematological cancer, Nucala® for severe eosinophilic asthma and several products in early clinical development, including apabetalone for atherosclerosis, abexinostat for lymphoma and a cathepsin S inhibitor for psoriasis. As VP Research at Affymax, Peter supported the CMC, translational, non-clinical safety and immunogenicity aspects of the development of Omontys®, a once a month pegylated peptidic erythropoietic agent, for the treatment of anemia. Peter is co-author on over 120 papers and co-inventor on 34 issued patents.[/fusion_modal][fusion_modal name=”Robert” title=”Robert Spiegel, M.D. FACP” size=”large” background=”” border_color=”” show_footer=”yes” class=”” id=””]Robert Spiegel joined the LAM Therapeutics Scientific Advisory Board in April, 2013. Dr. Spiegel is the founder of Spiegel Consulting LLC, an independent advisory firm to pharmaceutical and biotechnology companies, and serves as a Senior Advisor to Warburg Pincus and BioPontis Alliance. He retired in 2009 as Chief Medical Officer at Schering-Plough. With 25+ years experience in the pharmaceutical industry, he played a significant role in the development of novel biologics and drugs as Senior VP of Worldwide Clinical Research. Prior to joining Schering-Plough, Dr. Spiegel was an Assistant Professor, Department of Medicine, NYU Medical Center. Dr. Spiegel obtained his M.D. from the University of Pennsylvania and a B.A., cum laude, from Yale University. He received his training in Medical Oncology as a Research Associate at the National Cancer Institute, NIH. He currently serves on the Boards of numerous biotechnology companies and nonprofits and is an Assistant Professor of Medicine at Cornell Weill Medical College and Associate Fellow in the University of Pennsylvania Center for Bioethics.[/fusion_modal][fusion_modal name=”Brendan” title=”Brendan Manning, Ph.D.” size=”large” background=”” border_color=”” show_footer=”yes” class=”” id=””]Brendan Manning joined the LAM Therapeutics Scientific Advisory Board in May, 2013. Dr. Manning is a Professor in the Department of Genetics and Complex Diseases at the Harvard School of Public Health (HSPH). His doctoral training was in the laboratory of Michael Snyder at Yale University, where he worked basic mechanisms of cell division. Following receipt of his Ph.D. in 2000, Dr. Manning joined the laboratory of Lewis Cantley at Harvard Medical School for his postdoctoral research. During this time, he identified the tuberous sclerosis complex (TSC) tumor suppressor as the molecular connection between the PI3K and mTOR pathways, thereby linking a signaling pathway activated in the majority of human cancers to a nutrient-sensing pathway that controls cell growth and metabolism. In 2004, Dr. Manning joined the faculty of the then newly established Department of Genetics and Complex Diseases at HSPH to continue research at the interface of cancer and metabolism. He serves on multiple editorial boards, the Scientific Advisory Board of the LAM Foundation, and the Science and Medical Committee of the Tuberous Sclerosis Alliance.[/fusion_modal][fusion_modal name=”Lawrence” title=”Lawrence Melvin, Ph.D.” size=”large” background=”” border_color=”” show_footer=”yes” class=”” id=””]Larry Melvin joined the LAM Therapeutics Scientific Advisory Board in November, 2013. Dr. Melvin has over 30 years of experience in drug discovery and development with Pfizer, Amgen, Myogen and Gilead. Dr. Melvin has a broad background in medicinal chemistry including the areas of inflammation, respiratory, cardiovascular, central nervous system and antibacterial. He has initiated and led successful drug discovery programs yielding compounds for clinical study and participated in successful development programs such as Letairis™ for pulmonary arterial hypertension. Dr. Melvin earned his Ph.D. in synthetic organic chemistry from the University of Wisconsin and completed postdoctoral studies in organic synthesis at Harvard University. He is co-author or inventor on over 60 publications and over 60 issued US patents.[/fusion_modal][fusion_modal name=”Joel” title=”Joel S. Bader, Ph.D.” size=”large” background=”” border_color=”” show_footer=”yes” class=”” id=””]Joel Bader joined the LAM Therapeutics Scientific Advisory Board in December, 2014. Dr. Bader is an Associate Professor in the Department of Biomedical Engineering, Johns Hopkins University, and is Interim Director of the High-Throughput Biology Center at the Johns Hopkins School of Medicine. He is a member of the Institute of Computational Medicine and the Institute of Genetic Medicine, and he holds a secondary appointment in the Department of Computer Science at Johns Hopkins. Prior to joining Johns Hopkins in 2003, Dr. Bader was Director of Bioinformatics at CuraGen Corporation, where he co-invented the 454 Genome Sequencer with LAM founder Dr. Jonathan Rothberg and helped lead the generation of proteome-scale protein-protein interaction maps for yeast, fruit fly, and human. Dr. Bader’s research at Johns Hopkins focuses on systems biology, synthetic biology, and human disease genetics. Dr. Bader is co-founder of Neochromosome, Inc., a synthetic biology company. He received his Ph.D. in 1992 from U.C. Berkeley, where he was an NSF Predoctoral Fellow, and received an NSF CAREER Award while at Johns Hopkins.[/fusion_modal][fusion_modal name=”JoelDudley” title=”Joel Dudley, Ph.D.” size=”large” background=”” border_color=”” show_footer=”yes” class=”” id=””]Joel Dudley joined the LAM Therapeutics Scientific Advisory Board in December, 2014. Dr. Dudley is Assistant Professor of Genetics and Genomic Sciences and Director of Biomedical Informatics at the Icahn School of Medicine at Mount Sinai in New York City. Dr. Dudley obtained his PhD in Biomedical Informatics from Stanford University School of Medicine, where he developed research in the areas of genomic medicine and translational bioinformatics. Prior to Mount Sinai, he held positions as Co-founder and Director of Informatics at NuMedii, Inc. and Consulting Professor of Systems Medicine in the Department of Pediatrics at Stanford University. His current research is focused towards solving key challenges in precision medicine through the development and application of translational and biomedical informatics methodologies. His lab publishes in the areas of bioinformatics, genomic medicine, personal genomics, as well as drug and biomarker discovery. His previous work with co-authors describing a novel systems based approach for computational drug repositioning was featured in the print edition of the Wall Street Journal, and earned designation as the NHGRI Director’s Genome Advance of the Month. Dr. Dudley is co-author of the book Exploring Personal Genomics from Oxford University Press, and he was recently named by Fast Company magazine as one of the 100 Most Creative People in Business (2014).[/fusion_modal][fusion_modal name=”Paul” title=”Paul Beckett, Ph.D.” size=”large” background=”” border_color=”” show_footer=”yes” class=”” id=””]
Paul Beckett joined LAM Therapeutics as Senior Director, Drug Discovery in August, 2013. He is an internationally experienced medicinal chemist and drug discovery leader with a background in emerging biotech/pharmaceutical companies. He played a prominent role in the establishment of British Biotech Pharmaceuticals, the UK’s first publicly traded biotech company, where he contributed extensively to the discovery of the matrix metalloproteinase inhibitor BB-2516 (Marimastat, reached Phase III clinical trials in cancer) and the antibacterial agent BB-83698 (first-in-class to enter human clinical trials). As Director of Chemistry at Cara Therapeutics, he built the medicinal chemistry department from inception and initiated the discovery of a novel series of orally bioavailable, peripherally restricted, cannabinoid receptor agonists for the treatment of pain, as well as overseeing several other early stage projects. Most recently with the Institutes for Pharmaceutical Discovery, Paul was involved in multiple successful projects including the discovery of a novel series of arginase inhibitors with broad therapeutic potential. He is named as inventor on over 40 issued US patents and is an author on 35 scientific publications.
[/fusion_modal][fusion_modal name=”Rothberg” title=”Jonathan Rothberg” size=”large” background=”” border_color=”” show_footer=”yes” class=”” id=””]
Dr. Jonathan Rothberg is the Recipient of the National Medal of Technology and Innovation for inventing high-speed, “Next-Gen” DNA sequencing, the nation’s highest honor for technological achievement, bestowed by the president of the United States. He founded 454 Life Sciences, bringing to market the first new method for sequencing genomes since Sanger and Gilbert won the Nobel Prize in 1980. Dr. Rothberg sequenced the first individual human genome (James Watson’s Genome, Nature), and with Svante Paabo initiated the Neanderthal Genome Project. Under his leadership, 454 undertook the first deep sequencing of a cancer, helped understand the mystery behind the disappearance of the honey bee, uncovered a new virus killing transplant patients, and elucidated the extent of human variation—work recognized by Science magazine as the breakthrough of the year for 2007. The New England Journal described Dr. Rothberg’s innovation as “The New Age of Molecular Diagnostics”, Science magazine called it one of the top 10 breakthroughs for 2008. Dr. Rothberg went on to invent semiconductor chip-based sequencing, and sequenced Gordon Moore (Moore’s law, Nature), ushering in the age of the $1,000 Genome. In addition to founding 454 Life Sciences and Ion Torrent, Dr. Rothberg Founded CuraGen Corporation, Clarifi, RainDance Technologies, 4Catalyzer, Butterfly Network, Lam Therapeutics, and Hyperfine Research.
Dr. Rothberg was born in 1963 in New Haven, Connecticut. He earned a B.S. in chemical engineering from Carnegie Mellon University and an M.S., M.Phil, and Ph.D. in biology from Yale University. He is the first person to be named a World Economic Forum’s Technology Pioneer four separate times, is an Ernst and Young Entrepreneur of the Year, received The Wall Street Journal’s First Gold Medal for Innovation, Nature Methods First Method of the Year Award, The Irvington Institute’s Corporate Leadership Award in Science, the Connecticut Medal of Technology, the DGKL Biochemical Analysis Prize, and an Honorary Doctorate of Science from Mount Sinai School of Medicine. Jonathan is a member of the National Academy of Engineering, the Connecticut Academy of Science and Engineering, is a trustee of Carnegie Mellon University, and an Adjunct Professor of Genetics at the Yale School of Medicine.
[/fusion_modal][fusion_modal name=”Fergus” title=”Gregg Fergus” size=”large” background=”” border_color=”” show_footer=”yes” class=”” id=””]
Gregg Fergus is the Chief Executive Officer and Chief Commercial Officer of 4Catalyzer, a Guilford, Connecticut-based startup accelerator dedicated to democratizing healthcare and maximizing societal impact. 4Catalyzer provides funding, support and mentoring, and has raised over $180 million for three of its healthcare startup companies. In addition to management, his responsibilities include hiring the technical and commercial teams and serving on the board of each startup.
Prior to joining 4Catalyzer, Gregg was the President and Chief Operating Officer of Ion Torrent, which pioneered an entirely new approach to DNA sequencing. In addition to directing and managing the company, Gregg was instrumental in raising $40 million in funding for Ion Torrent in 2009. In 2010, a year before the company launched its first product, Gregg negotiated the sale of Ion Torrent to Life Technologies for $725 million. Ion Torrent’s PGM sequencer became the fastest selling DNA sequencer in the world shortly after going to market and it generated $550 million in revenue over three years. The Ion Torrent division was worth $2.4 billion in the 2014 sale of Life Technologies to Thermo Fisher.
Gregg’s other previous executive roles include Executive in Residence at RW Baird/Baird Venture Partners, where he provided investment thesis, deal flow and industry contacts for the Life Science tools market, Chief Commercial Officer of Cellular Dynamics International and Senor Vice President of Sales and Global Operations at Affymetrix, Inc. When he’s not working, Gregg enjoys keeping fit and has competed in the IRONMAN Triathlon.
[/fusion_modal][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container hundred_percent=”yes” overflow=”visible” admin_toggled=”yes” admin_label=”Anchor – News”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_menu_anchor name=”news” class=”” /][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container hundred_percent=”no” equal_height_columns=”no” menu_anchor=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”center center” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” admin_label=”Container – News” admin_toggled=”no”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”11% 0px 11% 0px” margin_top=”” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no”][fusion_text]
News
[/fusion_text][fusion_builder_row_inner][fusion_builder_column_inner type=”1_6″ layout=”1_6″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_text]
Date
[/fusion_text][/fusion_builder_column_inner][fusion_builder_column_inner type=”2_3″ layout=”2_3″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_text]
Title / Expand to Read More
[/fusion_text][/fusion_builder_column_inner][fusion_builder_column_inner type=”1_6″ layout=”1_6″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”10px” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_text]
View
[/fusion_text][/fusion_builder_column_inner][/fusion_builder_row_inner][fusion_builder_row_inner][fusion_builder_column_inner type=”1_6″ layout=”1_6″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”0px” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_text]
November 30, 2017
[/fusion_text][/fusion_builder_column_inner][fusion_builder_column_inner type=”2_3″ layout=”2_3″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”20px” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_accordion type=”toggles” boxed_mode=”” border_size=”1″ border_color=”” background_color=”” hover_color=”” divider_line=”” icon_size=”” icon_color=”” icon_boxed_mode=”” icon_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=””][fusion_toggle title=”LAM Therapeutics Announces Progress of its Clinical Portfolio with FDA Clearance of New Trial in Leukemia and Advancement of Clinical Trial in Lymphoma” open=”no”]
GUILFORD, Conn.—Nov. 30, 2017– LAM Therapeutics (LAM), a 4Catalyzer company, advanced its clinical portfolio with the U.S. Food and Drug Administration (FDA) clearance of LAM’s Investigational New Drug (IND) application for LAM-003 in leukemia patients. LAM-002 has transitioned into Phase 2 as a single agent and in combination with other therapies for treatment of B-cell non-Hodgkin lymphoma (B-NHL). Read more…
[/fusion_toggle][/fusion_accordion][/fusion_builder_column_inner][fusion_builder_column_inner type=”1_6″ layout=”1_6″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”10px” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_imageframe image_id=”1012″ style_type=”none” stylecolor=”” hover_type=”liftup” bordersize=”” bordercolor=”” borderradius=”” align=”none” lightbox=”no” gallery_id=”” lightbox_image=”” alt=”View PDF” link=”https://lamtherapeutics.com/wp-content/uploads/2017/12/2017-11-29-LAM-Press-Release-FINAL.pdf” linktarget=”_blank” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=””]https://lamtherapeutics.com/wp-content/uploads/2017/12/PDF-button-40px-copy.png[/fusion_imageframe][/fusion_builder_column_inner][/fusion_builder_row_inner][fusion_builder_row_inner][fusion_builder_column_inner type=”1_6″ layout=”1_6″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”0px” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_text]
March 30, 2017
[/fusion_text][/fusion_builder_column_inner][fusion_builder_column_inner type=”2_3″ layout=”2_3″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”20px” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_accordion type=”toggles” boxed_mode=”” border_size=”1″ border_color=”” background_color=”” hover_color=”” divider_line=”” icon_size=”” icon_color=”” icon_boxed_mode=”” icon_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=””][fusion_toggle title=”LAM Therapeutics Announces Close of $58M Series C Financing and New Clinical Program in Cancer” open=”no”]
GUILFORD, Conn., March 30, 2017 – LAM Therapeutics, a 4Catalyzer company that develops drugs for cancer and rare diseases, announced that it has closed $58M in Series C financing. 4Catalyzer is Connecticut’s premier biotechnology incubator and has raised over a quarter billion dollars to support its current class of startups.
LAM Therapeutics’ strategy
LAM accelerates drug development by integrating genomics and proteomics technologies with deep learning artificial intelligence (AI) to match clinical drugs to new indications. Read more…
[/fusion_toggle][/fusion_accordion][/fusion_builder_column_inner][fusion_builder_column_inner type=”1_6″ layout=”1_6″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”10px” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_imageframe image_id=”1012″ style_type=”none” stylecolor=”” hover_type=”liftup” bordersize=”” bordercolor=”” borderradius=”” align=”none” lightbox=”no” gallery_id=”” lightbox_image=”” alt=”View PDF” link=”https://lamtherapeutics.com/wp-content/uploads/2017/12/2017-03-30-LAM-Press-Release-FINAL.pdf” linktarget=”_blank” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=””]https://lamtherapeutics.com/wp-content/uploads/2017/12/PDF-button-40px-copy.png[/fusion_imageframe][/fusion_builder_column_inner][/fusion_builder_row_inner][fusion_builder_row_inner][fusion_builder_column_inner type=”1_6″ layout=”1_6″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”0px” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_text]
February 2, 2016
[/fusion_text][/fusion_builder_column_inner][fusion_builder_column_inner type=”2_3″ layout=”2_3″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”20px” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_accordion type=”toggles” boxed_mode=”” border_size=”1″ border_color=”” background_color=”” hover_color=”” divider_line=”” icon_size=”” icon_color=”” icon_boxed_mode=”” icon_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=””][fusion_toggle title=”LAM Therapeutics Closes $40M in Financing and Announces Two Clinical-Stage Programs in Rare Diseases and Cancer” open=”no”]
GUILFORD, Conn. –Feb. 2, 2016– LAM Therapeutics closed a $40 million financing to accelerate the development of therapeutics for the treatment of rare diseases and cancer. LAM Therapeutics is a 4Catalyzer company. 4Catalyzer is Connecticut’s premier biotechnology startup incubator and with this financing has raised a total of a quarter billion dollars to support its current class of startups. Read more…
[/fusion_toggle][/fusion_accordion][/fusion_builder_column_inner][fusion_builder_column_inner type=”1_6″ layout=”1_6″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”10px” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_imageframe image_id=”1012″ style_type=”none” stylecolor=”” hover_type=”liftup” bordersize=”” bordercolor=”” borderradius=”” align=”none” lightbox=”no” gallery_id=”” lightbox_image=”” alt=”View PDF” link=”https://lamtherapeutics.com/wp-content/uploads/2017/12/2016-02-02-LAM-Press-Release-FINAL.pdf” linktarget=”_blank” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=””]https://lamtherapeutics.com/wp-content/uploads/2017/12/PDF-button-40px-copy.png[/fusion_imageframe][/fusion_builder_column_inner][/fusion_builder_row_inner][fusion_builder_row_inner][fusion_builder_column_inner type=”1_6″ layout=”1_6″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”0px” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_text]
June 06, 2013
[/fusion_text][/fusion_builder_column_inner][fusion_builder_column_inner type=”2_3″ layout=”2_3″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”20px” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_accordion type=”toggles” boxed_mode=”” border_size=”1″ border_color=”” background_color=”” hover_color=”” divider_line=”” icon_size=”” icon_color=”” icon_boxed_mode=”” icon_alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=””][fusion_toggle title=”LAM Therapeutics launches operations to develop drugs for rare lung disease, Lymphangioleiomyomatosis (LAM)” open=”no”]
Guilford, CT – June 6, 2013 – LAM Therapeutics, the first biopharmaceutical company dedicated to identifying and developing drugs for the treatment of Lymphangioleiomyomatosis (LAM), announced today that it has closed a Series A financing with private investors to launch operations. Proceeds from the financing will be used for identification of clinical stage drugs with potential activity against LAM and to conduct clinical trials. Read more…
[/fusion_toggle][/fusion_accordion][/fusion_builder_column_inner][fusion_builder_column_inner type=”1_6″ layout=”1_6″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” padding_top=”10px” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no” border_position=”all”][fusion_imageframe image_id=”1012″ style_type=”none” stylecolor=”” hover_type=”liftup” bordersize=”” bordercolor=”” borderradius=”” align=”none” lightbox=”no” gallery_id=”” lightbox_image=”” alt=”View PDF” link=”https://lamtherapeutics.com/wp-content/uploads/2017/12/2013-06-06-LAM-Press-Release-FINAL.pdf” linktarget=”_blank” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=””]https://lamtherapeutics.com/wp-content/uploads/2017/12/PDF-button-40px-copy.png[/fusion_imageframe][/fusion_builder_column_inner][/fusion_builder_row_inner][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container hundred_percent=”yes” overflow=”visible” admin_toggled=”yes” admin_label=”Anchor – Contact”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_size=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” last=”no” hover_type=”none” link=”” border_position=”all”][fusion_menu_anchor name=”contact” class=”” /][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Container – Contact Form” hundred_percent=”no” hundred_percent_height=”no” hundred_percent_height_scroll=”no” hundred_percent_height_center_content=”yes” equal_height_columns=”no” menu_anchor=”” hide_on_mobile=”no” class=”” id=”” background_color=”#f4f6f8″ background_image=”” background_position=”left top” background_repeat=”repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”0px” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”11%” padding_right=”” padding_bottom=”11%” padding_left=”” admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”1_2″ layout=”1_2″ spacing=”yes” center_content=”no” link=”” target=”_self” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” hover_type=”none” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding_top=”50px” padding_right=”” padding_bottom=”50px” padding_left=”” margin_top=”” margin_bottom=”” animation_type=”” animation_direction=”left” animation_speed=”0.1″ animation_offset=”” last=”no”][fusion_text]
Contact Us
We would love to hear from you.
[/fusion_text][fusion_code]W2NvbnRhY3QtZm9ybS03IGlkPSZxdW90OzUmcXVvdDsgdGl0bGU9JnF1b3Q7Q29udGFjdCBmb3JtIDEmcXVvdDtd[/fusion_code][/fusion_builder_column][fusion_builder_column type=”1_2″ layout=”1_2″ spacing=”” center_content=”no” link=”” target=”_self” min_height=”none” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” undefined=”” background_repeat=”no-repeat” hover_type=”none” border_size=”” border_color=”” border_style=”solid” border_position=”all” padding_top=”50px” padding_right=”0px” padding_bottom=”50px” padding_left=”0px” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no”][fusion_map address=”530 Old Whitfield St., Guilford, CT 06437″ type=”roadmap” width=”100%” height=”520px” zoom=”12″ scrollwheel=”no” scale=”yes” zoom_pancontrol=”yes” animation=”yes” popup=”yes” map_style=”custom” overlay_color=”#6796bf” infobox_content=”LAM Therapeutics, Inc.
530 Old Whitfield St.
Guilford, CT 06437″ infobox=”custom” infobox_text_color=”#ffffff” infobox_background_color=”rgba(000,000,000,.4)” icon=”https://lamtherapeutics.com/wp-content/uploads/2017/06/LAM_Logo_Icon_Only_57x57px.png” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” /][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container][fusion_builder_container admin_label=”Container – Map” hundred_percent=”yes” equal_height_columns=”yes” menu_anchor=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”” background_position=”left top” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”0px” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”0px” padding_right=”0px” padding_bottom=”0px” padding_left=”0px” admin_toggled=”yes”][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_2″ spacing=”” center_content=”no” link=”” target=”_self” min_height=”” hide_on_mobile=”no” class=”” id=”” background_color=”” background_image=”https://lamtherapeutics.com/wp-content/uploads/2017/11/4C-Campus_1500px.jpg” background_position=”left top” undefined=”” background_repeat=”no-repeat” hover_type=”zoomin” border_size=”” border_color=”” border_style=”solid” border_position=”all” padding_top=”0px” padding_right=”0px” padding_bottom=”0px” padding_left=”0px” margin_top=”0px” margin_bottom=”0px” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no”][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container]